Dr Jamie Kitt, Dr Andrew Kelion and Dr Elizabeth Orchard.
Department of Cardiology, Oxford Heart Centre, John Radcliffe Hospital, Oxford, United Kingdom
A 46-year-old civil servant was referred to us by a local district general hospital (DGH) in April 2018. He had been admitted in October 2017 after developing rapid palpitations whilst in the shower. He attended his local Emergency Department and was found to be in atrial flutter with 2:1 block and a ventricular rate of 140 beats per minute. He was seen by the on-call cardiologist and started on Bisoprolol and Apixaban. He was referred to the local Electrophysiologist. An A&E bedside scan (GE V-scan) done locally was ‘reportedly normal’ and he went on to have a successful flutter (cavo-tricuspid isthmus) ablation in December 2017.
At our clinic in June 2018, he reported no further palpitations, but mentioned fatigue with reduced exercise tolerance compared to early 2017. On reflection, he felt that he had always struggled to keep up with his peers at school and university. He had no other past medical history of note, and was a non-smoker who drank only occasional alcohol. He took only Apixaban 5mg bd.
On examination, oxygen saturation was 99% on room air. Pulse rate was 66 and regular, with normal character and volume, and blood pressure was 115/60. Jugular venous pulse was normal. He had wide fixed splitting of the second heart sound, and a pan-systolic murmur loudest at the lower left sternal edge. The chest was clear.
12-lead electrocardiogram (ECG) demonstrated sinus rhythm, with partial right bundle branch block (RBBB) and a QRS duration of 100ms.
Question 1: What is the likely diagnosis at this point based on the current information?
- Ventricular septal defect (VSD)
- Atrio-ventricular septal defect (AVSD)
- Mitral regurgitation
- Atrial septal defect (ASD)
- Pulmonary stenosis
Answer: 4. Atrial septal defect (ASD). The combination of long-standing exercise intolerance, an atrial arrhythmia, wide fixed splitting of the second heart sound and RBBB morphology on the ECG is highly suggestive of an ASD1.
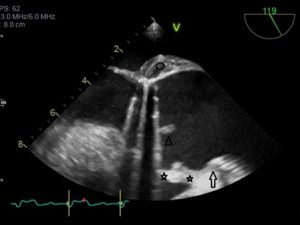
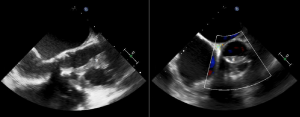
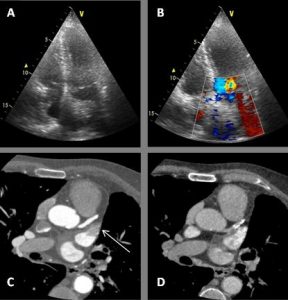
At follow up in the DGH, he had eventually had a formal trans-thoracic echo and a dilated right heart was noted as well as an atrial septal defect which prompted the referral to us. A transthoracic echocardiogram (TTE) at our clinic was reported as a primum ASD with a left to right shunt (Figure 1). There was also moderate mitral regurgitation due to prolapse of the anterior mitral valve leaflet (AMVL) with thickening of the leaflet tips (Figure 2). The right ventricle (RV) was moderately dilated, with severe bi-atrial dilatation.
Figure 1. Trans-thoracic echo apical 4 chamber view A : Severely enlarged left atrium B: Severely dilated RA with evidence of RA pressure overload at end systole (arrow) C: RV dilatation (albeit in snap-shot at end-systole) D (arrow): Elongated AMVL with prolapse
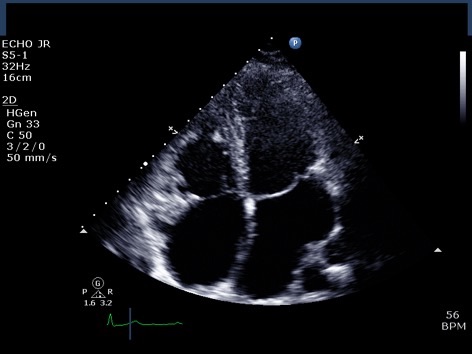
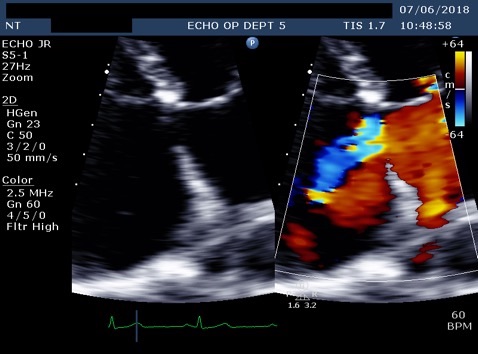
Figure 2. A: Zoomed apical 4 chamber of the inta-atrial septum showed a defect (measured as 1.3cm) reported as a primum ASD. B: Corresponding colour flow Doppler through the defect confirming left to right shunt
Transoesophageal echocardiography (TOE) was performed a few weeks later, and the images were reviewed at our Adult Congenital Heart Disease (ACHD) Multidisciplinary Team (MDT) Meeting. The study was felt to show a primum ASD, with an accompanying bubble study which appeared to show a left to right shunt (Figures 3A and 3B). However, the atrial defect itself was not clearly visualised, so cardiac magnetic resonance (CMR) imaging was advised to define the anatomy more precisely.
Figure 3A. Trans-oesophageal echocardiohgram (TOE) mid-oesophageal at 71 degrees focusing on the intra-atrial septum (IAS) A: Severely enlarged right atrium. B: IAS with small primum ASD suspected but unable to clearly visualise at any angulation C: Off axis tri-leaflet aortic valve D: Partial view of a dilated RV
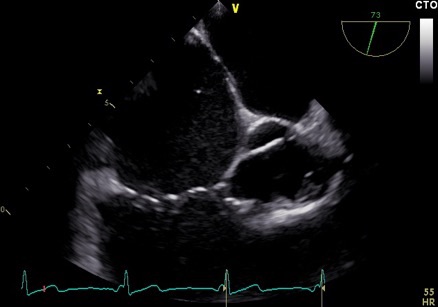
Figure 3B. Bubble study during TOE at 3rd cardiac cycle after injection into left arm (unaware of left sided SVC at this time) consistent with large left to right shunt.
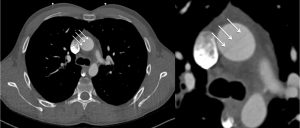
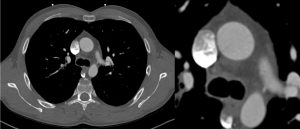
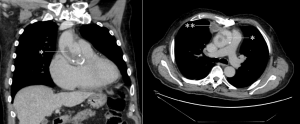
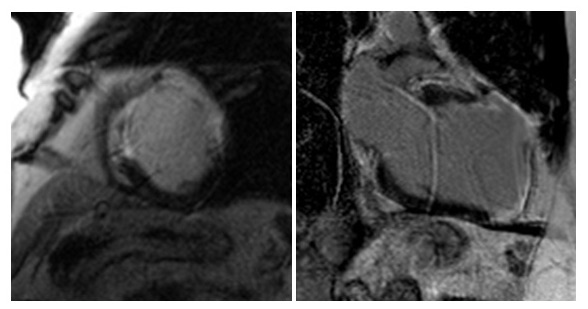
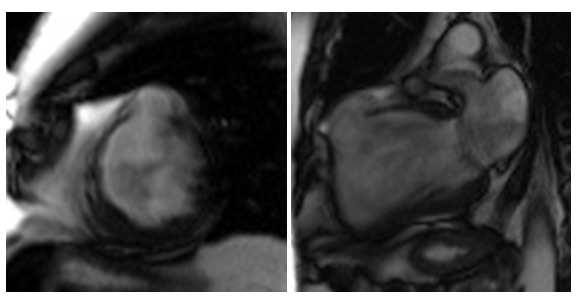
CMR was performed in July 2018, including a detailed atrial stack and a twist angiogram in addition to the standard set of sequences. The study reportedly showed a moderate secundum ASD located antero-inferiorly (Figure 4A and 4B), resulting in a 2:1 left to right shunt. A comment was made that no primum ASD was seen. There was a mildly dilated RV when indexed to his body surface area (235mls) with normal RV function, normal LV function, and mild mitral regurgitation. Normal pulmonary venous (PV) drainage was noted, but a persistent left-sided superior vena cava (SVC) was seen on the twist angiogram though it was unclear whether this was draining into the right atrium (RA).
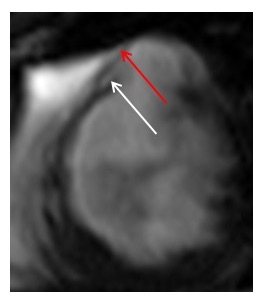
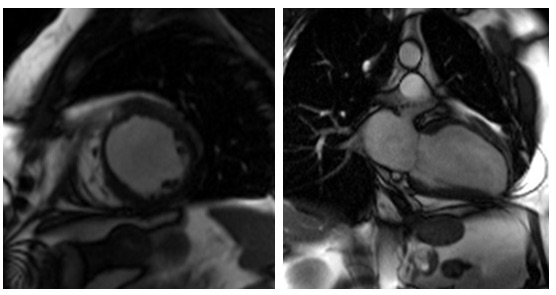
Figure 4. Cardiac MRI performed using Siemens 1.5T magnet. 4A Atrial stack A:Severely dilated left atrium. B: Severely dilated right atrium. C (arrow): Atrial Septal defect visualised reported as moderate sized secundum atrial septal defect (ASD) measuring (21x17mm) which is located very antero-inferiorly. Flows subsequently confirmed Qp:Qs 2:1. 4B Horizontal long axis (HLA) view also showing significant atrial septal defect (arrow) and D: moderately dilated RV
Question 2: What is the commonest variation in the thoracic venous system?
- Anomalous drainage of right sided superior vena cava (SVC) to the left atrium (LA)
- Persistent left-sided SVC (bilateral SVCs aka SVC duplication)
- Isolated left sided SVC
Answer: 2. Persistent left-sided SVC, is the most common congenital venous anomaly in the chest.
It accounts for 10% of thoracic vein anomalies and is present in 0.3-0.5% of the general population2. It is only seen in isolation in 10% of cases since in the vast majority of cases (82-90%) a normal (but small) right-sided SVC is also present, and a persistent bridging vein (left brachiocephalic vein) is seen in 25-35% of cases.
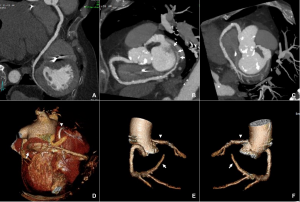
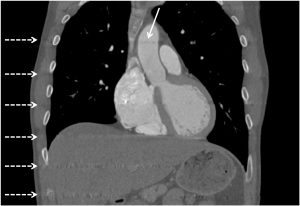
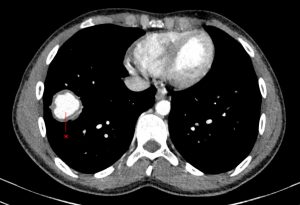
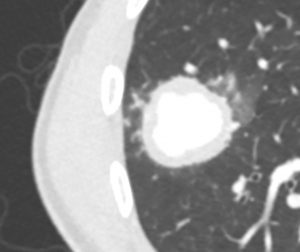
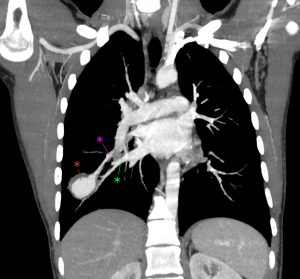
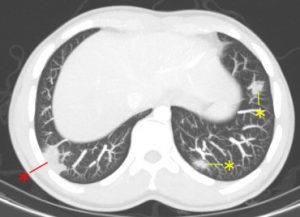
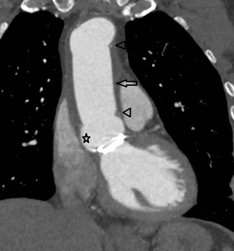
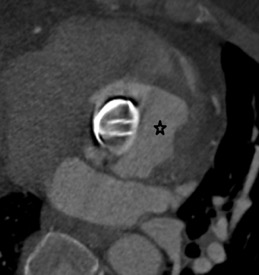
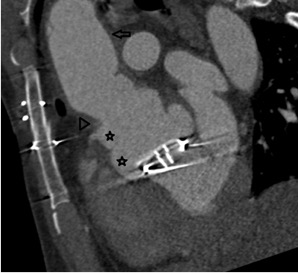
On further discussion at the ACHD MDT Meeting in August 2018, there was still no agreement on the exact anatomy despite TTE, TOE with a bubble study, and CMR. He was duly referred for a gated cardiac X-ray computed tomographic (CT) study, which resolved the confusion. CT demonstrated a right-sided SVC and inferior vena cava (IVC) draining normally into the RA. The persistent left-sided SVC (figure 5B in Powerpoint) drained into a completely de-roofed coronary sinus and LA (Figure 5), creating a large effective “ASD”. An additional small secundum ASD was also evident. PV drainage was normal. The right ventricle and both atria were dilated. The coronary arteries were normal.
Figure 5 . Gated Cardiac CT which was performed on a GE Revolution with 16cm detector, using Omnipaque 350 50ml at 5ml/s, saline 50ml at 5ml/s. No metoprolol or GTN was administered. Prospective gating at 70-80% as well as 0-100% at 20% current. 100kVp. Heart rate 64bpm. DLP 223mGy.cm.
5A: A: Deroofed coronary sinus creating large ‘ASD’ as well as a B: Secundum ASD and 5B shows C: Left sided SVC draining into De-roofed coronary sinus and Left atrium (LA).
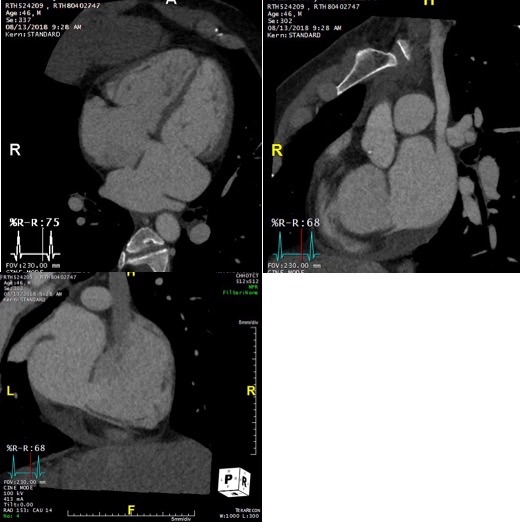
Question 3: What is the commonest associated congenital lesion seen with a left sided SVC
- Atrial septal defect (ASD)
- Ventricular septal defect (VSD)
- Coarctation of the aorta
- Pulmonary stenosis
- Anomalous pulmonary venous return
Answer: 1. ASD. Congenital heart defects are present in 4.4% of patients with left-sided SVC3. ASDs are the most common by far but it can also occur with a single atrium, VSDs, Tetralogy of Fallot, coarctation of the aorta, anomalous pulmonary venous drainage and pulmonary stenosis. These should be thus searched for if a left sided SVC is present. There are a number of possible drainage sites. Left-sided SVCs are often functionally insignificant with 92% draining into coronary sinus since venous return from the head, neck and upper limbs is delivered to the right atrium 3. In the other 8%, although drainage into the LA results in a right to left shunt, it is usually not large enough to cause cyanosis or symptoms 3but, in this case the coronary sinus ASD and second secundum ASD resulted in a large left to right shunt.
Discussion:
This case highlights the importance of comprehensive cardiological assessment of patients with new onset atrial arrhythmias, including a detailed TTE, particularly in young adults, before jumping to treat the rhythm disorder. It also highlights the importance of searching for other lesions once one congenital lesion has been identified. Moreover, it emphasises the strengths and limitations of the various imaging modalities used in assessment of complex congenital heart disease. Echocardiography and CMR provide excellent functional information. However, when the anatomy is uncertain, gated cardiac CT offers the unparalleled ability to perform extensive reorientation of a true three-dimensional dataset with high spatial resolution. There is no requirement for images to have been acquired in precisely the right plane, as for echo and CMR. Modern CT scanners such as the GE REVOLUTION also allow easy dose modulation across the entire cardiac cycle, so that even LV and RV systolic function can be calculated to complement the anatomical assessment.
References:
- S Martin, E Shapiro and M Mukherjee. Atrial Septal Defects – Clinical Manifestations, Echo Assessment, and Intervention. Clin Med Insights Cardiol. 2014; 8 (Suppl 1): 93–98. PMCID: PMC4373719
- Padhani AR, Hale HL. Mediastinal venous anomalies: potential pitfalls in cancer diagnosis. Br J Radiol. 1998; 71 (847): 792-8. Br J Radiol
- Pretorius PM, Gleeson FV. Case 74: right-sided superior vena cava draining into left atrium in a patient with persistent left-sided superior vena