Author: Mahon C, Kempny A and Semple T
Royal Brompton and Harefield Hospital, London, United Kingdom.
Educational points:
Three-dimensional volume rendered reformatting with virtual stent implantation can assist in predicting the anatomical result of a catheter-based repair of sinus venosus atrial septal defect (SVASD) and partial anomalous pulmonary venous drainage.
Case Report
A seventy-year-old male with dyspnoea on exertion and new onset pedal oedema was referred for further management of a SVASD. He had presented 10 years prior with atrial fibrillation while undergoing pre-assessment for an elective knee replacement. Echocardiography at the time revealed a SVASD. The patient declined open cardiac surgical repair at the time. He had an uneventful knee replacement and was managed medically from a cardiac perspective. He was referred to our centre for consideration of novel transcatheter repair.
On examination his oxygen saturations were 98% on room air. His body mass index was 29kg/m2, blood pressure 125/80mmHg, resting heart rate 79 beats per minute and irregularly irregular. His jugular venous pressure was elevated with prominent C-V waves. Chest auscultation revealed a grade 2/6 pansystolic murmur and clear lungs. He had a right ventricular heave.
An electrocardiogram (ECG) demonstrated atrial fibrillation at a rate of 72bpm with right bundle branch block and P-pulmonale in leads II, III and AVF.
Repeat transthoracic echocardiography demonstrated a 14 x 20mm SVASD with a left-to-right shunt at rest, severe right ventricle dilation with preserved function and a dilated inferior vena cava with <50% inspiratory collapse.
A right heart catheter carried out confirmed left-to-right shunt. Cardiac output was calculated using Fick’s indirect method. The transpulmonary blood flow (Qp) was 9.7L/min, the transsystemic blood flow (Qs) was 4.5 L/min and the Qp/Qs was 2.2. The PVR was normal on invasive assessment at 2.1 woods units (see table 1).
A single-phase ECG triggered high pitch dual source spiral CT acquisition was performed using a weight-based CT angiography protocol. Images were reconstructed at 0.75mm slice thickness at an interval of 0.5mm, and reformatted using Aquarius iNtuition, Terarecon, Durham NC.
In addition to the SVASD, the CT revealed two PVs anomalously draining to the SVC. The right upper pulmonary vein (RUPV) drained into the SVC immediately above the right atrium (RA) junction. The right middle pulmonary vein (RMPV) drained below the RUPV directly to the RA. The right lower PV and all left PVs drained to the left atrium (LA) (figure 1 a-f). Curved planar reformats suggested that a 7 cm long stent could be used to redirect pulmonary venous return also demonstrated via virtual implantation of a stent (see image 1 images a,e and f).
A balloon test was performed prior to stent insertion. SVC balloon inflation with RUPV and RMPV angiogram was performed to confirm SVASD occlusion, and re-routing of the PVs without obstruction.
The patient underwent successful closure of the SVASD and rerouting of the RUPV and RMPV to the LA (figure 1 g-h).
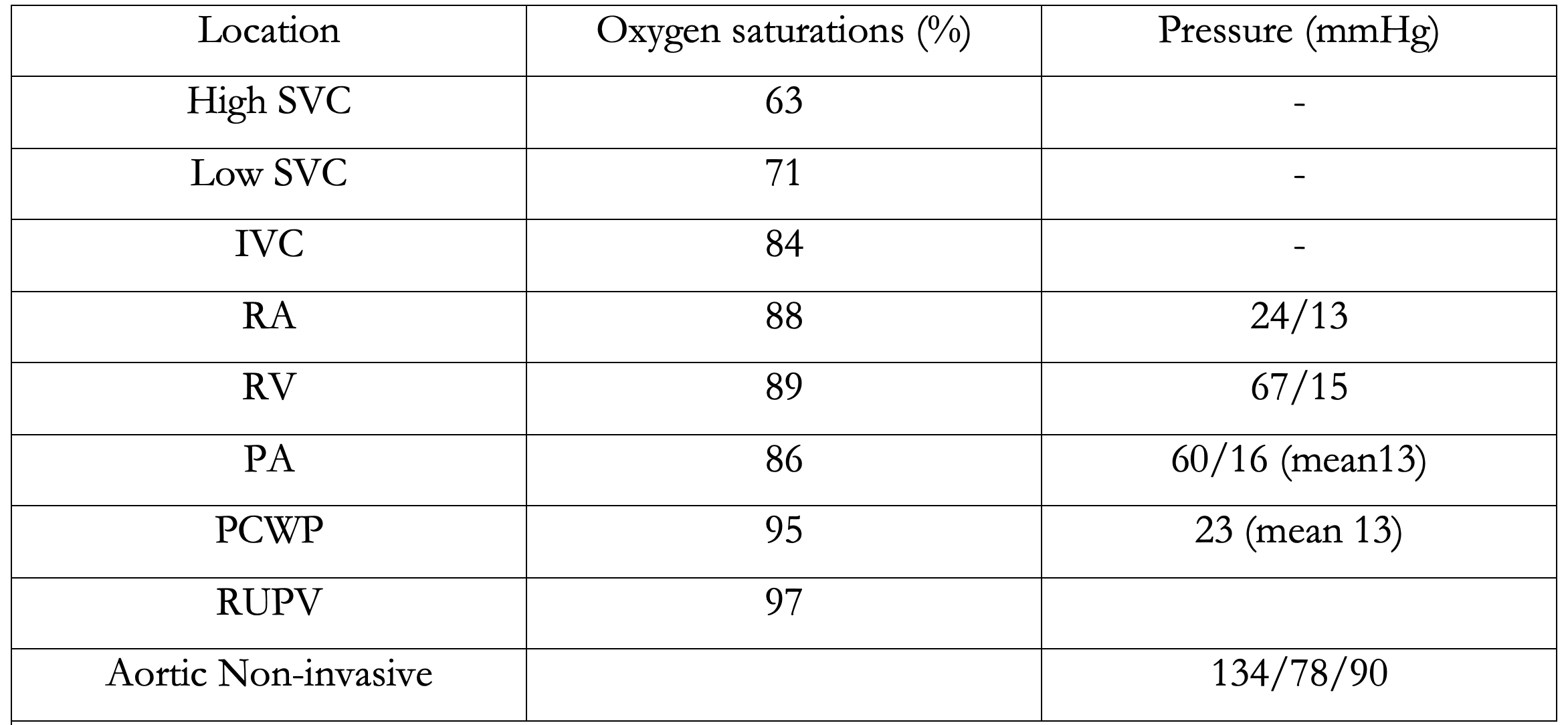
Table 1: Right heart catheter results
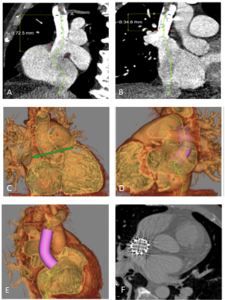
Figure 1 a-b shows a curved planar reformat of the SVC from the level of the azygous to the RA with the SVASD and PAPVD; c shows the three-dimensional volume rendered image of the SVASD; d-e shows the virtual stent and g shows the actual anatomical result post SVASD stent insertion.
Discussion
ASD is a common congenital heart defect with the prevalence of an isolated defect ranging from 0.5 to 2.5 cases per 1,000 live births (1). The resulting shunt depends on the type and size of the defect, the pressures in the RA and LA, the function of the atrioventricular valves and the diastolic properties of the ventricles (2). Many patients are asymptomatic at presentation with an ASD (2).
Closure of an ASD with signs of a significant shunt is associated with improved clinical outcomes as compared to conservative therapy, regardless of age (3). Signs of a significant shunt include right heart dilation or a documented increased Qp/Qs> 1.5. There is increasing evidence to support early ASD closure to improve morbidity (2, 4). The additional volume load from the shunt may lead to right heart dilation and associated progressive tricuspid regurgitation, increased risk of arrhythmia, and right ventricle dysfunction if untreated (2). In rare cases Eisenmenger’s may occur (2). Our patient was a candidate for intervention with a Qp/Qs of 2.2. He had right heart dilation with preserved function, severe tricuspid regurgitation, and atrial fibrillation in the absence of increased pulmonary vascular resistance on invasive assessment.
Surgical correction is the standard of care and, until recently, was the only treatment option (5,6). The incidences of complications are low with surgical intervention and include the general morbidity of sternotomy and cardiopulmonary bypass as well as specific complications, such as sinus node dysfunction, pulmonary venous obstruction, and occlusion of the SVC (7). Our patient was reluctant for open cardiac surgical intervention, and the novel minimally invasive approach was considered appropriate. The transcatheter approach using a covered stent deployed in the SVC-RA junction was first published by Garg et al in 2014 (8). Since then the technique has been adapted and modified by others (9, 10, 11). The covered stent replaces the deficient posterior wall of the SVC, thereby closing the SVASD redirecting any anomalous PVs into the LA behind the stent (10).
SVASD is well known to be associated with PAPVD (10). PAPVD can be more complex than a single RUPV or RMPV anomaly entering near the SVC-RA junction or within the RA itself (12). Multiple anomalous PVs or accessory PVs may be present and anomalous PVs on the left side may drain to other locations (7 and 12). Careful selection of patients before transcatheter SVASD closure involves meticulous assessment of the size and position of the PVs relative to the azygous vein and the SVASD prior to stent deployment (7). Both CT and cardiac magnetic resonance imaging can provide a 3-D dataset of the PV anatomy. CT acquisition is quicker with a better special resolution and is currently the preferred modality.
The anomalous PVs need to be directed behind the covered stent to the LA without the stent obstructing the pulmonary venous pathway. Where the junction of the anomalous PV is adjacent to the SVASD, a compromise may be needed between complete closure of the SVASD accompanied by narrowing of the pulmonary venous pathway; leaving a clinically negligible shunt with a widely patent PV; or reconsidering surgery (7). Anomalous PVs that enter high into the SVC may need to remain draining to the SVC. A similar approach is often taken during surgical correction (7). If re-routing is necessary, and/or the risk of PV obstruction is high with stent deployment, then surgery should be considered. In the case presented the CT revealed two anomalous PVs and offered a clear delineation of the PV insertion and anatomic relationship to the SVASD near the RA junction. Superimposition of the stent suggested closure of the SVASD and re-routing of the PAPVD would not cause PV obstruction and could successfully re-route the PVs to the LA. This CT reconstruction with the stent simulation can provide useful information in the decision pathway as to whether to proceed with a transcatheter approach, but cannot replace the requirement for balloon interrogation. The test occlusion will continue to be performed prior to stent deployment, and where there is suggestion of PV obstruction during balloon interrogation of the defect a decision needs to be made whether to continue of abandon the procedure (7).
Conclusion
Careful patient selection before transcatheter SVASD closure and meticulous assessment of the PVs is critical to a successful outcome without complication. CT 3D volume rendered images allow simulation of the stent anchoring positions, aiding decision making for whether to proceed the transcatheter intervention.
References:
- van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos-Hesselink JW. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011 Nov 15;58(21):2241-7. doi: 10.1016/j.jacc.2011.08.025. PMID: 22078432.
- Kempny A, Gatzoulis MA. Percutaneous repair of sinus venosus ASD: the end of congenital cardiac surgery? EuroIntervention. 2018 Oct 20;14(8):843-845. doi: 10.4244/EIJV14I8A150. PMID: 30339128
- Attie F, Rosas M, Granados N, Zabal C, Buendía A, Calderón J. Surgical treatment for secundum atrial septal defects in patients >40 years old. A randomized clinical trial. J Am Coll Cardiol. 2001 Dec;38(7):2035-42. doi: 10.1016/s0735-1097(01)01635-7. PMID: 11738312.
- Gatzoulis MA, Freeman MA, Siu SC, Webb GD, Harris L. Atrial arrhythmia after surgical closure of atrial septal defects in adults. N Engl J Med. 1999 Mar 18;340(11):839-46. doi: 10.1056/NEJM199903183401103. PMID: 10080846.
- P. Iyer, K. Somanrema, S. Pathak, P.Y. Manjunath, S. Pradhan, S. Krishnan Comparative study of single- and double-patch techniques for sinus venosus atrial septal defect with partial anomalous pulmonary venous connection J Thorac Cardiovasc Surg, 133 (2007), pp. 656-659
- D. Stewart, F. Bailliard, A.M. Kelle, C.L. Backer, L. Young, C. Mavroudis Evolving surgical strategy for sinus venosus atrial septal defect: effect on sinus node function and late venous obstructionAnn Thorac Surg, 84 (2007), pp. 1651-1655
- Rosenthal E, Qureshi SA, Jones M, Butera G, Sivakumar K, Boudjemline Y, Hijazi ZM, Almaskary S, Ponder RD, Salem MM, Walsh K, Kenny D, Hascoet S, Berman DP, Thomson J, Vettukattil JJ, Zahn EM. Correction of sinus venosus atrial septal defects with the 10 zig covered Cheatham-platinum stent – An international registry. Catheter Cardiovasc Interv. 2021 Jul 1;98(1):128-136. doi: 10.1002/ccd.29750. Epub 2021 May 7. PMID: 33909945.
- Garg, H. Tyagi, A.S. Radha Transcatheter closure of sinus venosus atrial septal defect with anomalous drainage of right upper pulmonary vein into superior vena cava—an innovative technique Catheter Cardiovasc Interv, 84 (2014), pp. 473-477
- Crystal MA, Vincent JA, Gray WA. The wedding cake solution: A percutaneous correction of a form fruste superior sinus venosus atrial septal defect. Catheter Cardiovasc Interv. 2015 Dec 1;86(7):1204-10. doi: 10.1002/ccd.26031. Epub 2015 May 22. PMID: 26011715. Hansen HJ, Duong P, Jivanji SG, Jones M., Kabir S, Butera . Transcatheter Correction of Superior Sinus Venosus Atrial Septal Defects as an Alternative to Surgical Treatment. JACC 2020 ;75(11)1266-1278
- Hansen HJ, Duong P, Jivanji SG, Jones M., Kabir S, Butera . Ttranscatheter Correction of Superior Sinus Venosus Atrial Septal Defects as an Alternative to Surgical Treatment. JACC 2020 ;75(11)1266-1278
- Riahi M, Velasco Forte MN, Byrne N, Hermuzi A, Jones M, Baruteau AE, Valverde I, Qureshi SA, Rosenthal E. Early experience of transcatheter correction of superior sinus venosus atrial septal defect with partial anomalous pulmonary venous drainage. EuroIntervention. 2018 Oct 20;14(8):868-876. doi: 10.4244/EIJ-D-18-00304. PMID: 30012542.
- Hatipoglu S, Almogheer B, Mahon C, Houshmand G, Uygur B, Giblin GT, Krupickova S, Baksi AJ, Alpendurada F, Prasad SK, Babu-Narayan SV, Gatzoulis MA, Mohiaddin RH, Pennell DJ, Izgi C. Clinical Significance of Partial Anomalous Pulmonary Venous Connections (Isolated and Atrial Septal Defect Associated) Determined by Cardiovascular Magnetic Resonance. Circ Cardiovasc Imaging. 2021 Aug;14(8):e012371. doi: 10.1161/CIRCIMAGING.120.012371. Epub 2021 Aug 13. PMID: 34384233.





