Williams MG1; Harries I2; Kassimis G3; Bucciarelli-Ducci, C1
1Department of Cardiology, Bristol Heart Institute, United Kingdom
2Department of Radiology, Bristol Royal Infirmary, United Kingdom
3Department of Cardiology, Cheltenham General Hospital, United Kingdom
Case report
A previously healthy 34 year old man presented acutely to a district general hospital with chest pain. An electrocardiogram confirmed sinus rhythm with an inferior ST elevation myocardial infarction. His risk factors included a family history of premature coronary artery disease (father, aged 35 years), hypercholesterolaemia (total cholesterol 9 mmol/litre) and elevated body mass index (29 kg/m2). He was taken immediately for a coronary angiogram. This revealed a dominant right coronary artery which was occluded at the posterior descending artery (PDA) (Figure 1a). He also had chronic total occlusions of his circumflex, distal LAD and first diagonal arteries (Figure 1b). Primary percutaneous coronary intervention (PCI) was then performed. Balloon angioplasty of his occluded PDA was done as the culprit lesion and the patient was started on standard secondary prevention medication. Outpatient stress perfusion cardiac magnetic resonance imaging (CMR) was arranged to guide further potential revascularisation strategies.
CMR demonstrated low normal left ventricular systolic function with an ejection fraction of 59%. There was hypokinesia of the mid and apical anterior walls and of the basal inferior wall. Stress images were obtained using a standard adenosine protocol and showed extensive inducible myocardial perfusion defects affecting the basal and mid-anterior, anterolateral, inferolateral walls, mid and apical inferior walls (8 segments in total) (See Figures 2 and 3). There was a small focus of subendocardial late gadolinium enhancement (LGE) of the basal inferior segment (Figure 4). If technically feasible, revascularisation of the circumflex and left anterior descending territories was advised on prognostic grounds.
The patient underwent elective PCI to his circumflex coronary artery with an excellent result (Figure 5). Technical challenges precluded revascularisation of the diagonal and distal LAD territories and so aggressive secondary prevention and anti-anginal therapy have been commenced.
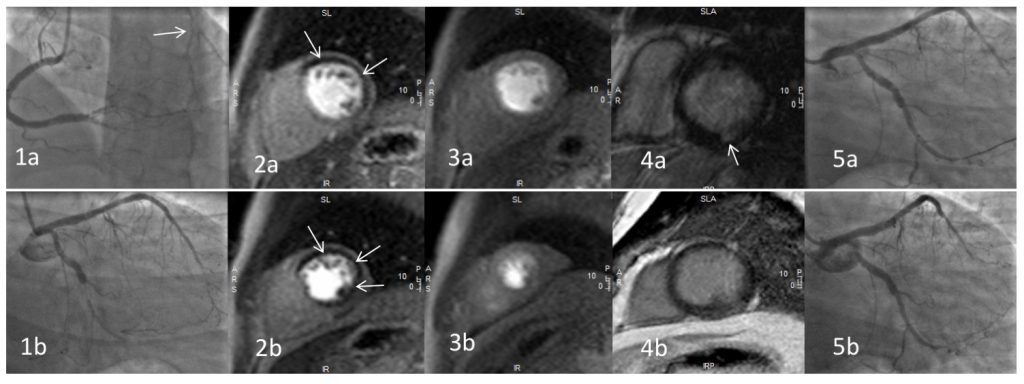
Figure 1. Coronary angiography of the right (1a) and left (1b) coronary arteries; note collateralisation to the chronically occluded circumflex (arrowed)
Figure 2. T1-weighted basal (2a) and mid ventricular (2b) short-axis stress perfusion images demonstrating an extensive inducible perfusion defect (arrowed), predominantly affecting the left anterior descending and left circumflex coronary artery territories.
Figure 3. T1-weighted basal (3a) and mid ventricular (3b) short-axis rest perfusion images demonstrating normal perfusion.
Figure 4. T1-weighted basal (4a) and mid ventricular (4b) short-axis late gadolinium enhancement sequences demonstrating minimal late enhancement in the inferior segment (arrowed).
Figure 5. Percutaneous coronary intervention to the circumflex artery (5a) with an excellent final result (5b).
Multiple choice questions
1. According to current ESC guidelines, what is considered to be a significant ischaemic burden that warrants revascularisation?
A) 5%
B) 10%
C) 15%
D) 20%
E) 25%
Answer B)
Current guidelines suggest that in patients with stable coronary artery disease, a proven large area of ischaemia (≥ 10% of the left ventricle) on non-invasive testing warrants revascularisation on prognostic grounds1. However, recent evidence has suggested that an ischaemic burden of >1.5 segments on cardiac MRI is a strong predictor of poor outcome and this may be reflected in future guidelines2.
2. In patients with multi vessel disease after primary PCI, what level of evidence is quoted by the 2017 ESC STEMI guidelines for the role of cardiac MRI to assess for myocardial ischaemia and viability to guide complete revascularisation?
A) It is not recommended by the ESC
B) I
C) IIa
D) IIb
E) III
Answer D) IIb. The ESC STEMI guidelines (2017)3 state that CMR may be used after primary PCI to assess for ischaemia and viability including in multivessel coronary artery disease.
References
1. Gilles Montalescot (France), Udo Sechtem (Germany), Stephan Achenbach (Germany), Felicita Andreotti (Italy), Chris Arden (UK), Andrzej Budaj (Poland), Raffaele Bugiardini (Italy), Filippo Crea (Italy), Thomas Cuisset (France), Carlo Di Mario (UK), J. Rafael Ferreira (Portugal), Bernard J. Gersh (USA), Anselm K. Gitt (Germany), Jean-Sebastien Hulot (France), Nikolaus Marx (Germany), Lionel H. Opie (South Africa), Matthias Pfisterer (Switzerland), Eva Prescott (Denmark), Frank Ruschitzka (Switzerland), Manel Sabate ́ (Spain), Roxy Senior (UK), David Paul Taggart (UK), Ernst E. van der Wall (Netherlands), Christiaan J.M. Vrints (Belgium). 2013 ESC guidelines on the management of stable coronary artery disease. European Heart Journal (2013) 34, 2949–3003
2. Gabriella Vincenti, Pier Giorgio Masci, Pierre Monney, Tobias Rutz, Sarah Hugelshofer, Mirdita Gaxherri, Olivier Muller, Juan F. Iglesias, Eric Eeckhout, Valentina Lorenzoni, Cyril Pellaton, Christophe Sierro, Juerg Schwitter: Stress Perfusion CMR in Patients With Known and Suspected CAD. JACC: Cardiovascular Imaging May 2017, 10 (5) 526-537; DOI: 10.1016/j.jcmg.2017.02.006
3. Borja Ibanez, Stefan James, Stefan Agewall, Manuel J Antunes, Chiara Bucciarelli-Ducci, Héctor Bueno, Alida L P Caforio, Filippo Crea, John A Goudevenos, Sigrun Halvorsen, Gerhard Hindricks, Adnan Kastrati, Mattie J Lenzen, Eva Prescott, Marco Roffi, Marco Valgimigli, Christoph Varenhorst, Pascal Vranckx, Petr Widimský, ESC Scientific Document Group ; 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC), European Heart Journal, ehx393, https://doi.org/10.1093/eurheartj/ehx393