Authors
Dr Amir Masood Rafie Manzelat, Dr Matthew Morgan, Dr Chris Critoph
Royal Bournemouth Hospital
Case presentation
An adult female with a long-standing history of supraventricular tachyarrhythmias was followed up for progressive left ventricular hypertrophy (LVH), initially attributed to hypertensive heart disease. She had experienced recurrent palpitations since her mid-thirties and was subsequently diagnosed with paroxysmal atrial fibrillation and atrial flutter, requiring long-term anticoagulation. She also reported exertional dyspnoea consistent with heart failure with preserved ejection fraction.
Electrocardiography demonstrated sinus rhythm with a normal PR interval and no evidence of pre-excitation. While PR interval shortening in the absence of pre-excitation has been described as an early electrical red flag in Fabry disease, a normal PR interval does not exclude the diagnosis.
The patient had a background history of asthma and non-muscle-invasive bladder cancer, diagnosed in 2022 (grade 2 pTa with associated carcinoma in situ), which was managed with transurethral resection followed by intravesical BCG therapy and cystoscopic surveillance. These comorbidities were not felt to contribute to the cardiomyopathic phenotype.
Imaging Findings
Cardiac magnetic resonance imaging performed in December 2020 demonstrated global concentric left ventricular hypertrophy, with a basal septal thickness of 1.5 cm at end diastole. Left ventricular volumes and systolic function were preserved, with an ejection fraction of 68%. There was no evidence of left ventricular outflow tract obstruction or systolic anterior motion of the mitral valve.
Late gadolinium enhancement imaging revealed focal mid-wall pathological enhancement involving the basal inferolateral wall. Native myocardial T1 mapping demonstrated markedly reduced myocardial T1 values, measuring 788 ms (reference range 950–1050 ms). These imaging findings were considered highly suggestive of Fabry disease.
Following referral to a specialist inherited cardiac disease centre, the diagnosis of Fabry disease was confirmed and enzyme replacement therapy with agalsidase beta was initiated.
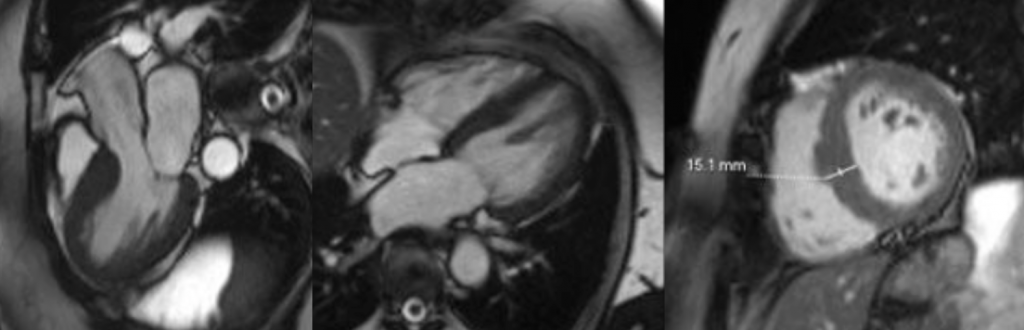
Cine cardiac MRI images
Short-axis and long-axis cine images demonstrating global concentric left ventricular hypertrophy with basal septal thickness measuring approximately 1.5 cm at end diastole, without evidence of left ventricular outflow tract obstruction or systolic anterior motion of the mitral valve.
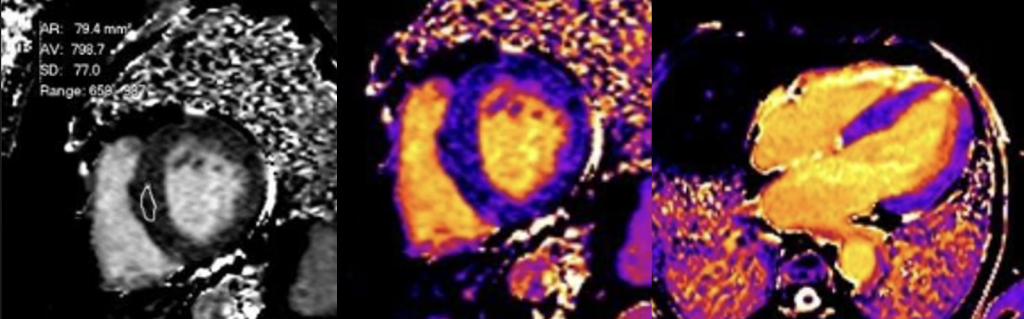
Native T1 mapping images
Short-axis and long-axis native T1 maps demonstrating diffusely reduced myocardial T1 values throughout the left ventricle, consistent with intracellular lipid accumulation.
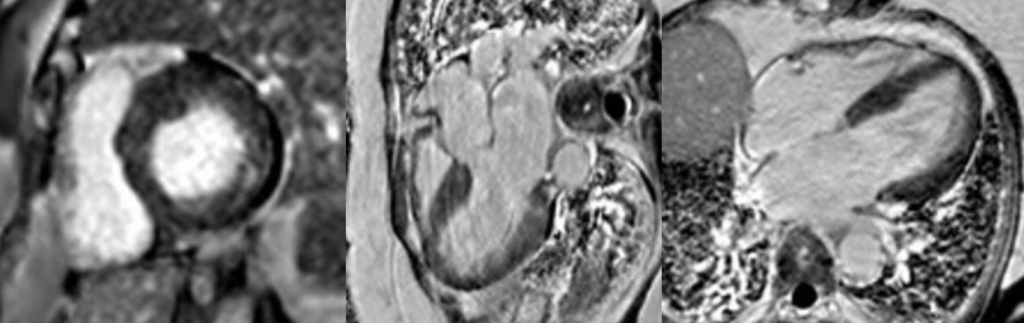
Late gadolinium enhancement images
Short-axis and long-axis late gadolinium enhancement sequences demonstrating focal mid-wall pathological enhancement involving the basal inferolateral wall.
Discussion
Fabry disease is a rare X-linked lysosomal storage disorder caused by deficiency of α-galactosidase A, resulting in intracellular accumulation of glycosphingolipids within multiple organs, including the myocardium. Cardiac involvement is common and represents a major contributor to morbidity, frequently manifesting as concentric left ventricular hypertrophy, diastolic dysfunction, arrhythmias, and heart failure with preserved ejection fraction.
Diagnosis is often delayed due to significant clinical and imaging overlap with more prevalent causes of myocardial hypertrophy, particularly hypertensive heart disease and hypertrophic cardiomyopathy. This challenge is amplified in female patients, in whom phenotypic expression may be variable. While electrocardiographic abnormalities such as PR interval shortening without pre-excitation may represent an early red flag for Fabry disease, these findings are neither sensitive nor specific and may be absent, as demonstrated in this case.
Cardiac magnetic resonance imaging plays a pivotal role in the diagnosis of Fabry cardiomyopathy. The combination of concentric LVH, focal mid-wall late gadolinium enhancement affecting the basal inferolateral wall, and markedly reduced native myocardial T1 values represents a characteristic imaging signature. Reduced native T1 reflects intracellular lipid accumulation and allows confident differentiation from infiltrative cardiomyopathies such as amyloidosis, which typically demonstrate elevated native T1 values, and from sarcomeric hypertrophic cardiomyopathy, which lacks a consistent enhancement pattern.
The presence of late gadolinium enhancement reflects myocardial fibrosis and is associated with increased arrhythmic risk and adverse outcomes. In this case, the imaging findings provided a unifying explanation for the patient’s long-standing atrial arrhythmias and heart failure symptoms and enabled timely initiation of enzyme replacement therapy.
Key Learning Point
Low native myocardial T1 values combined with inferolateral mid-wall late gadolinium enhancement on cardiac MRI are highly suggestive of Fabry disease and should prompt referral for specialist assessment in patients with unexplained concentric left ventricular hypertrophy, even in the absence of supportive echocardiographic or electrocardiographic red flags.
References
Umer M, Ghosh RK, Kalra A, et al. Cardiac magnetic resonance imaging in Fabry disease: diagnostic and prognostic implications. Front Cardiovasc Med. 2023;10:1123456.
Ponsiglione A, Gambardella M, Sica G, et al. Cardiovascular magnetic resonance native T1 mapping in Anderson–Fabry disease: a systematic review and meta-analysis. J Cardiovasc Magn Reson. 2022;24(1):67.
Figliozzi S, Imbriaco M, D’Errico A, et al. Effects of enzyme replacement therapy on cardiac magnetic resonance findings in Fabry disease. Radiology. 2024;310(2):e230456.
Dougherty S, Baig S, Elliott PM. Cardiac manifestations of Fabry disease. Nat Rev Cardiol. 2025;22(1):45–60.
Pande S, Moon JC, Hughes DA. Fabry disease cardiomyopathy: pathophysiology, imaging, and treatment. Clin Cardiol. 2025;48(2):123–132.






