Author:
Dilan Sanli
University Hospital Southampton NHS Foundation Trust
Case Summary
A 31-year-old woman with a history of two previous uncomplicated pregnancies was scheduled for elective ovarian cyst removal last year. On the day of surgery, a cardiac murmur was incidentally detected, prompting further cardiac evaluation.
She denied any significant cardiac symptoms, including chest pain or exertional chest tightness. She described herself as not particularly physically active but otherwise in good general health. Her BMI was elevated at 34 kg/m², with no traditional cardiovascular risk factors. She reported occasional non-specific palpitations over the preceding 3–6 months, each episode lasting up to 48 hours, but denied presyncope, syncope, increasing fatigue, or lethargy. She was a lifelong non-smoker and abstained from alcohol. There was no history of hypertension, diabetes, renal, or hepatic disease.
On examination, cardiovascular findings were unremarkable — normal heart sounds with no audible murmur.
Investigations
Echocardiography (multiple transthoracic studies and one transoesophageal) revealed:
· Flow directed towards the main pulmonary artery
· Systolic and diastolic flow along the interventricular septum
· Markedly dilated right coronary artery (RCA)
· Left ventricle (LV) visually mildly dilated with mild systolic dysfunction for age
The findings were suspicious for either a coronary artery fistula or, more likely, anomalous left coronary artery arising from the pulmonary artery (ALCAPA), with extensive collateralization between the right and left coronaries.
A CT coronary angiogram confirmed the diagnosis:
· The left mainstem originated from the main pulmonary artery (MPA)
· The left coronary artery (LCA) was markedly dilated without plaque or stenosis
· The RCA was markedly dilated and tortuous, supplying multiple collaterals to the LCA
A cardiac MRI with stress perfusion showed:
· Overall normal LV size and function
· Minor focal hypokinesis and relative myocardial thinning at basal to mid anterior and anteroseptal segments
· No LV dilatation or hypertrophy
· No late gadolinium enhancement, indicating absence of prior infarction
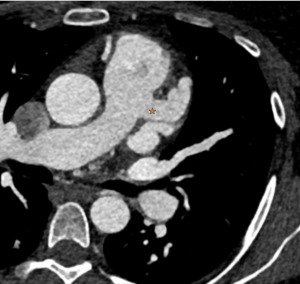
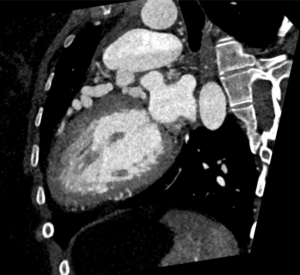
Figure 1: Demonstrates LAD, originates from the main Pulmonary Artery leading to dilated and tortuous left anterior descending (LAD) artery.
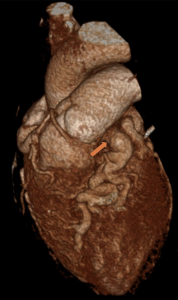
Figure 2: 3D demonstration of the main pulmonary origin of the LAD.
Figure 3: Demonstrates enlarged tortuous intercoronary collateral arteries along epicardial surface of heart.
Figure 4: Demonstrates a cross-sectional view of the coronary anatomy of the patient.
Figure 5: Take a note of the change of the contrast opacification from the right ventricular outflow tract into the main pulmonary artery due to reversal of flow.
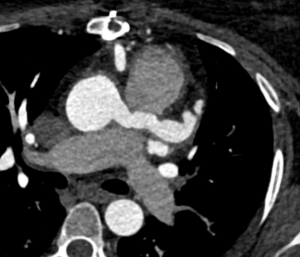
Figure 6: Following the corrective surgery, the left coronary arises from the ascending aorta and is tunnelled through the main pulmonary artery trunk.
Discussion:
Anomalous Left Coronary Artery from the Pulmonary Artery (ALCAPA) is a rare congenital cardiac anomaly, accounting for only 0.25–0.5% of all congenital heart defects.
In utero and for several weeks after birth, the coronary circulation is adequately perfused due to the high neonatal pulmonary vascular resistance (PVR) and the presence of the patent ductus arteriosus (PDA). Though the myocardium is perfused with desaturated blood from the pulmonary artery, this is adequate to maintain myocardial oxygen delivery and adequate ventricular function. With ductal closure and a progressive decline in PVR in the first two to three months of life, coronary flow reverses due to the lower pressure in the pulmonary circulation, producing a left-to-right shunt and a coronary steal phenomenon, resulting in decline in myocardial perfusion. Without sufficient collateralization from the RCA, this leads to myocardial ischemia, LV dysfunction, and infarction within weeks to months of life.
In infancy, ALCAPA typically presents as Bland-White-Garland syndrome, characterized by crying during feeds (angina equivalent), diaphoresis, tachypnoea, and grunting. Infants may feed briefly (“snackers”) and show signs of fatigue, pallor, or failure to thrive. In adults, presentation is variable; ranging from asymptomatic cases discovered incidentally, to exertional dyspnoea, arrhythmia, or sudden cardiac death.
ALCAPA is characterized by a chronically ischemic but potentially viable myocardium. If uncorrected, mortality is extremely high; however, survival into adulthood may occur in rare cases with well-developed collaterals. Chronic subendocardial ischemia and fibrosis increase the risk of sudden cardiac death due to ventricular arrhythmias.
Management
Diagnosis of ALCAPA constitutes an absolute indication for surgical correction, with the goal of establishing a two-coronary-artery system. The preferred approach is direct reimplantation of the LCA into the aorta. If this is not technically feasible, alternative options include creation of an intrapulmonary tunnel (Takeuchi procedure) or other revascularization techniques.
Teaching Points
· ALCAPA should be considered in any infant with dilated cardiomyopathy or myocardial ischemia without obstructive coronary disease.
· In adults, prominent coronary collaterals and an enlarged RCA should raise suspicion.
· CT and MRI provide detailed anatomic and functional assessment, complementing echocardiography.
· Early diagnosis and surgical revascularization are lifesaving and prevent irreversible myocardial damage.
QUIZ:
1) A 4-month-old infant with anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA), severe mitral regurgitation, and a dilated left ventricle with severely diminished ventricular function is admitted to the cardiac ICU in severe congestive heart failure. The mitral valve papillary muscles are noted to be echo-bright. In patients with ALCAPA, which of the following co-existing lesions is MOST LIKELY to be associated with a less severe degree of myocardial ischemia?
A. Mitral regurgitation
B. Atrial septal defect
C. Ventricular septal defect
D. Pulmonary stenosis
2) Which of the following is the most associated valvular abnormality in ALCAPA?
A. Mitral Regurgitation
B. Tricuspid Regurgitation
C. Aortic Regurgitation
D. Pulmonary stenosis
Answers:
- C – Explanation: Associated anomalies, such as a PDA or ventricular septal defect (VSD), lead to a gradual increase in left to right intracardiac shunt as PVR falls and thus, increased PVR related to increased blood flow. This may create a “protective” effect on the ventricular muscle by maintaining higher coronary perfusion pressure and myocardial oxygen delivery.
- A – Explanation: Myocardial ischaemia leads to LV dilatation which leads to mitral regurgitation.
References:
1. https://radiopaedia.org/articles/anomalous-left-coronary-artery-from-the-pulmonary-artery
2. Yu J, Ren Q, Liu X, et al. Anomalous left coronary artery from the pulmonary artery: Outcomes and management of mitral valve. Front Cardiovasc Med. 2022;9:953420. doi: 10.3389/fcvm.2022.953420
3. Cottrill CM, Davis D, McMillen M, O’Conner WN, Noonan JA, Todd EP. Anomalous Left Coronary Artery from the Pulmonary Artery: Significance of Associated Intracardiac Defects. J Am Coll Cardiol. 1985; 6: 237-242.
4. https://heart.bmj.com/content/83/1/e2