Authors: Tom Foster 1, Michelle C Williams 2, Marialena Gregoriades 1
Department of Radiology, Western General Hospital, Edinburgh
University of Edinburgh, Edinburgh
Case history:
An elderly patient was admitted with a several week history of fatigue and reduced mobility. They had previously undergone tissue AVR and CABG.
On examination they were noted to have splinter haemorrhages, Osler’s nodes and elevated inflammatory markers. This raised the suspicion of infective endocarditis (IE).
However, blood cultures were all negative and trans-thoracic echo did not demonstrate any vegetations. Trans-oesophageal echo was felt not to be indicated given negative blood cultures. A non-gated contrast enhanced computed tomography (CT) scan of the chest, abdomen and pelvis was performed to investigate infection of unknown origin.
CT images identified a 45mm filling defect extending from the aortic valve into the lumen of the ascending aorta (Fig. 1). The aorta was thick walled in keep with mural oedema, suggestive of aortitis. Anteriorly in the mediastinum, anterior to the aorta and posterior to the septum there was a separate walled off, enhancing collection.
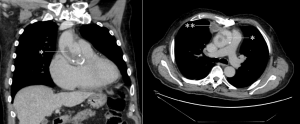
Figure 1. Coronal (left) and axial (right) CT images showing large filling defects within the ascending thoracic aorta (marked *). An enhancing collection is seen in the anterior mediastinum on the axial view (marked **).
The presence of vegetations arising from the aortic valve was subsequently confirmed on trans-oesophageal echo (Fig. 2).
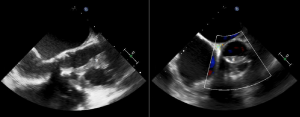
Figure 2. Trans-oesophageal echo showing large vegetations adjacent to the aortic valve (marked with *).
The patient was started on broad spectrum antibiotics and intravenous antifungal treatment. Further investigation with multiple blood cultures subsequently demonstrated fungal infection.
Discussion:
Infective endocarditis is infection of the inner lining of the heart, usually affecting the heart valves, with an incidence rate of approximately 1.7-6.2 cases per 100,000 patient years [1]. Despite being relatively uncommon, it is important to recognise early due to high rates of morbidity and mortality.
The most frequent aetiology is a bacterial infection, with Staphylococcus aureus and viridans group streptococci being the most common causative organisms. Other bacteria associated with endocarditis include coagulase-negative Streptococci and Enterococci, with a number of other organisms seen less frequently (such as the ‘HACEK’ group of organisms). Fungal endocarditis is less common, with Candida and Aspergillus species being the most frequent fungi identified.
Clinical presentation is variable and often linked to the underlying causative organism, with for example infective endocarditis with Staphylococcus aureus generally causes an acute presentation (<2 weeks) when compared to viridans group streptococci which are generally associated with a more subacute presentation (weeks to months). Signs and symptoms of infective endocarditis include:
- Fever and constitutional symptoms (e.g. fatigue, malaise, etc.) – these are seen in the vast majority of patients.
- Heart murmur – new or changing; this finding causes particular concern when associated with prosthetic valves.
- Immunological phenomena (e.g. Osler’s nodes on the hands/feet, Roth’s spots on the retina).
- Vascular phenomena (e.g. Janeway lesions on the hands/feet, septic emboli, splinter haemorrhages).
The modified Duke criteria (2004) can be used to diagnose infective endocarditis by assessing whether certain pathological or clinical diagnostic criteria are met. Clinical criteria involve elements of the patient history (e.g. predisposing factors), examination findings (e.g. pyrexia, Osler’s nodes and Janeway lesions), blood culture results and imaging findings. Imaging is most often in the form of echocardiography (both transthoracic and transoesophageal) but other modalities such as CT may be useful, as in this case. The European Society of Cardiology have more recently produced new modified diagnostic criteria in 2015 [2] recognise the value of cardiac CT and nuclear imaging in diagnosis.
This particular case demonstrates some of the difficulties in diagnosing infective endocarditis. Transthoracic echo in particular is limited as even large vegetations may not be identified. Prosthetic valves in particular can be challenging to image with transthoracic echo as metal artefact may obscure vegetations. It is therefore important to not be falsely reassured by negative blood cultures and a normal transthoracic echo.
While transoesophageal echo is the ‘gold standard’ imaging test for suspected infective endocarditis, this situation highlights the potential value of CT in the diagnosis. Some patients may be too frail or not able to tolerate transoesophageal echo. Large vegetations may be identified on non-gated imaging such as in this case, but for more subtle cases gated CT may be required. Many CT scans are performed to assess sepsis of unknown origin, therefore the heart should be an important review area in such cases.
It is also important to remember fungal endocarditis as a potential source of culture-negative endocarditis. Fungal endocarditis is a rare condition but is particularly associated with immunocompromise, long term broad-spectrum antibiotic use, central venous catheters and previous cardiac surgery [3]. It is becoming increasingly common in the intensive care setting and a high index of suspicion for fungal endocarditis is important in patients in this setting who fail to respond to antibiotic therapy [2]. Embolic events are common in fungal endocarditis, more so than in bacterial endocarditis and may complicate diagnosis and subsequent management. One literature review showed that over half of cases had evidence of embolic events at initial presentation [4].
Questions:
- What is the most common causative organism in fungal endocarditis?
- Aspergillus
- Candida
- Cryptococcus
- Histoplasma
- Tricosporon
- What is the approximate mortality rate of fungal endocarditis?
- 1-2 %
- 10 %
- 25 %
- 30 %
- >50 %
- Which of these factors is not recognised as a predictor of poor outcome in infective endocarditis?
- Large vegetations
- Low left ventricular ejection fraction
- Non-HACEK Gram-negative bacilli as the causative organism
- Premature mitral valve closure
- Viridans group streptococci as the causative organism
Answers:
- b- Candida spp is the most common causative organism, being responsible for roughly a quarter to a half of cases of fungal endocarditis, Aspergillus spp being responsible for approximately another quarter and other fungi including Histoplasma species making up the remaining cases [5].
- e- Fungal endocarditis is a serious condition and fatal in approximately 50 % of cases [2]. This is for a number of reasons:
- Difficulties in establishing a diagnosis – blood cultures are often negative. Clinical manifestations are similar to bacterial endocarditis and therefore important to consider fungal endocarditis in cases of infective endocarditis that do not respond to antibiotic therapy. Diagnosis is made post-mortem in a large number of cases [6].
- Difficulty in treatment – treatment requires potent antifungal agents (such as amphotericin B and caspofungin) and surgical management (if patient is fit) [2]. Treatment success rate with medical therapy alone is low, and risk of recurrence is high. Specialist local microbiologist input is vital.
- Associated comorbidities – fungal endocarditis often occurs in those with other underlying medical problems, such as those who are immunosuppressed.
- Lack of awareness – fungal endocarditis perhaps is less well recognised than bacterial endocarditis.
- e- Viridans group streptococci are associated with a more subacute presentation and better prognosis. All the other answers are predictors of poor outcome [2]. Premature mitral valve closure is a feature of elevated left ventricular diastolic pressure.
References:
[1] Beynon RP, Bahl VK, et al. Infective endocarditis. BMJ 2006; 333:334-9.
[2] Habib G, Lancellotti P, ESC Scientific Document Group et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). European Heart Journal 2015; 36(44): 3075–3128.
[3] Pierrotti LC, Baddour LM. Fungal endocarditis, 1995-2000. Chest 2002; 122(1): 302–10.
[4] Kalokhe AS, Rouphael N et al. Aspergillus endocarditis: a review of the literature. Int J Infect Dis 2010; 14: 1040–1047.
[5] Ellis ME, Al-Abdely H, et al. Fungal endocarditis: evidence in the world literature, 1965-1995. Clin Infect Dis 2001; 32:50.
[6] Seelig MS, Speth CP et al. Patterns of Candida endocarditis following cardiac surgery: Importance of early diagnosis and therapy (an analysis of 91 cases). Prog Cardiovasc Dis 1974; 17(2):125-60.





