Authors: Laura Duerden1, Tom Chance1, Jonathan Rodrigues2, Stephen Lyen1, Mark Hamilton1, Nathan Manghat1
Affiliations:
1 Department of Radiology, Bristol Royal Infirmary, United Kingdom
2 Department of Cardiothoracic Imaging, Toronto General Hospital, Toronto
Case
A 17 year-old girl presented with palpitations. She had no past medical history and no family history of cardiac disease. Clinical examination and initial investigations including trans-thoracic echocardiography showed no abnormality. 24-hour electrocardiograph holter monitoring revealed frequent ventricular ectopic beats.
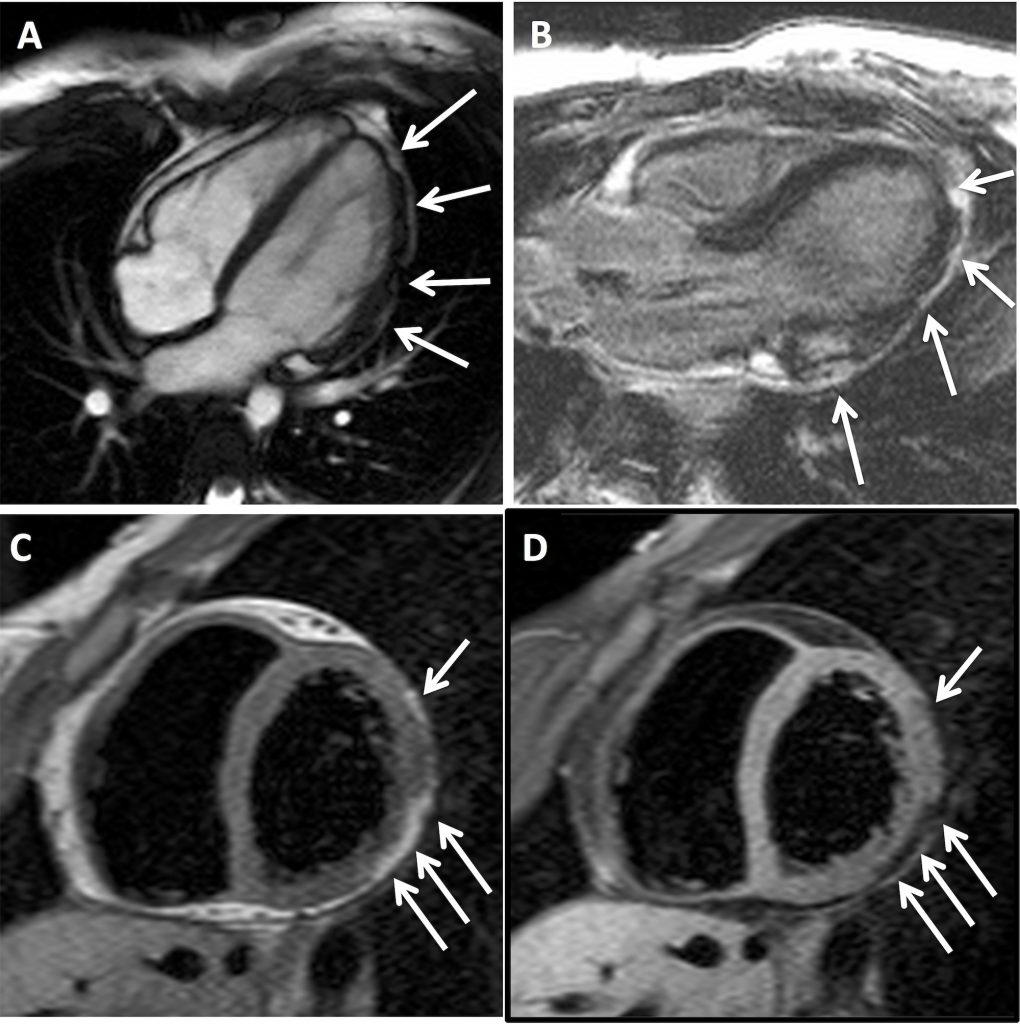
Owing to persistent symptoms, she was referred for cardiac magnetic resonance imaging (CMR). This showed mildly impaired left ventricular function, with hypokinesia and thinning / irregularity of the left antero-lateral and infero-lateral ventricular wall (Fig 1A). There was evidence of patchy epicardial late gadolinium enhancement in the lateral LV wall (Fig 1B) on the post-gadolinium sequence. No right ventricular abnormality was demonstrated. A provisional diagnosis of chronic myocarditis was made but the absence of a prolonged period of chest pain in her clinical history was felt to be unusual and an interval CMR was performed one year later. This showed nodular signal abnormality in the left ventricular lateral wall which was hyper-intense to myocardium and iso-intense to fat on T1-weighted sequences (Fig 1C) and the exhibited homogenous signal drop out with fat suppression techniques (Fig 1D), implying focal fibro-fatty infiltration.
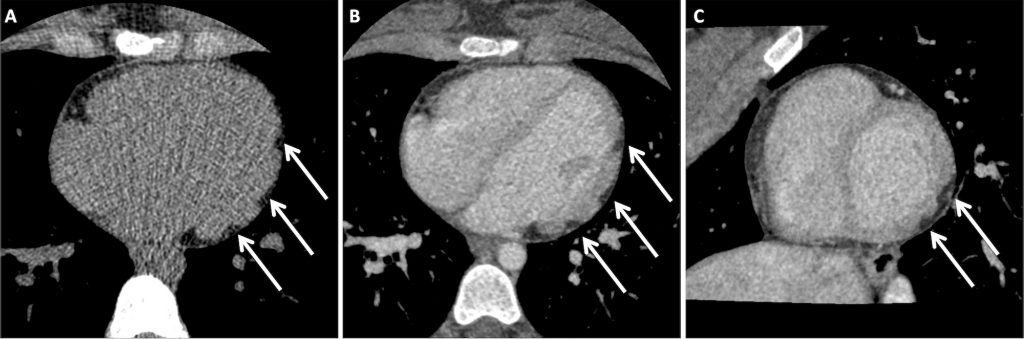
She went on to have a cardiac CT, performed with unenhanced and delayed phase imaging, to characterize the apparent nodular soft tissue seen on CMR. This confirmed abnormal lobulation of the left ventricular wall. There were multiple areas of focal epicardial thinning. The thin walled sections were invaginated by epicardial fat and blood vessels (Fig 2). The right ventricle was normal.
A diagnosis of arrythmogenic left ventricular cardiomyopathy (ALVC) was made. The patient was treated with bisoprolol to control palpitations and has remained well with no decline in left ventricular function. First-degree relatives have been screened with no other affected family members identified.
Figure legends
Figure 1
A) 4-chamber steady state free precession image demonstrating irregularities of left ventricular antero-lateral left ventricular wall (white arrows).
B) 3-chamber late gadolinium enhancement magnitude image demonstrating foci of epicardial enhancement in the infero-lateral left ventricular wall (white arrows).
C) Mid-ventricular short-axis T1-weighted image demonstrating epicardial foci of signal hyper-intense to myocardium and iso-intense to fat (white arrows).
D) Mid-ventricular short-axis T1-weighted fat-saturated image demonstrating homogenous signal drop out in the epicardial foci demonstrating high T1-weighted images in Figure 1C consistent with foci of epicardial fat.
Figure 2
A) Axial unenhanced ECG-gated cardiac CT image demonstrating foci of fat attenuation in the left ventricular lateral wall (white arrows).
B) Axial delayed ECG-gated cardiac CT image demonstrating foci of fat attenuation discrete to the enhancing myocardium in the left ventricular lateral wall (white arrows).
C) Reconstructed left ventricular short-axis delayed ECG-gated cardiac CT image demonstrating foci of fat attenuation discrete to the enhancing myocardium in the left ventricular lateral wall (white arrows).
Discussion
ALVC is an increasingly recognised condition, characterised by fibro-fatty replacement in the left ventricle wall. It is closely related to the more widely recognised arrythmogenic right ventricular cardiomyopathy, where the right ventricle is affected. The two disease patterns frequently co-exist within the same family and genes implicated in ARVC have also been identified in patients with ALVC.
Arrythmogenic cardiomyopathies are distinct from dilated cardiomyopathy (DCM) by predominately resulting in arrhythmia, rather than ventricular dysfunction. This is an important clinical diagnosis to be aware of as the major complication is sudden cardiac death due to malignant arrhythmia. Clinical heart failure is rarely seen.
Multiple-choice questions
Question 1:
Which of these is a CMR feature of myocarditis?
A) Focal, non-ischaemic lesion demonstrating late gadolinium enhancement
B) T2 signal decrease in myocardium
C) Decreased global myocardial early gadolinium enhancement ratio.
D) Intramyocardial fat
Answer:
A) Focal, non-ischaemic lesion demonstrating late gadolinium enhancement
The Lake Louise CMR criteria1 are used to diagnose myocarditis. If ≥ 2 of the following features are present, in the correct clinical context, then a diagnosis of myocarditis should be considered:
1) the presence of at least one, focal, non-ischaemic lesion demonstrating late gadolinium enhancement.
2) regional / global myocardial signal increase on T2-weighted images.
3) increased global myocardial early gadolinium enhancement ratio (EGEr).
Question 2:
Mutations in which of these genes is most frequently associated with arrythmogenic cardiomyopathy?
A) Collagen IV
B) Desmoplakin
C) Laminin
D) Plectin
Answer:
B) Desmoplakin
When a diagnosis of arrythmogenic cardiomyopathy is made, it is important to perform familial screening. This may include a genetic screen for genotype mutations in genes such as desmoplakin2 which may be implicated in the clinical phenotype in the proband.
Question 3:
What pattern of late gadolinium enhancement would be most consistent with a diagnosis of idiopathic dilated cardiomyopathy?
A) Epicardial
B) Mid-wall
C) Subendocardial
D) Transmural
Answer:
B) Mid-wall
Mid-wall LGE is associated with idiopathic dilated cardiomyopathy. However, mid-wall fibrosis is only seen in approximately 30% of individuals with idiopathic DCM3. Epicardial LGE is described in ARVC and ALVC. Subendocardial and transmural LGE are associated with ischaemic heart disease4.
Question 4:
Which of the following criteria was not part of the Modified Task Force Criteria for the diagnosis of arrhythmogenic right ventricular cardiomyopathy / dysplasia?
A ) Arrhythmias
B) Conduction / repolarization abnormalities
C) Endomyocardial biopsy
D) Family history
E) Global or region dysfunction
Answer:
C) Endoymocardial biopsy
For more information see the proposed modification of the Task Force Criteria5.
References
1 Friedrich MG, et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol. 2009; 53: 1475-87
2 Sen-Chowdhry S, et al. Left-dominant arrhythmogenic cardiomyopathy: an under-recognized clinical entity. J Am Coll Cardiol 2008; 52: 2176-87.
3 Assomull RG, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006; 48: 1977-85
4 Mahrholdt H, et al. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005; 26: 1461-74
5 Marcus FI, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J. 2010: 31: 806-14