An 85-year-old woman with chronic obstruction pulmonary disease was referred to the Heart Team for consideration of transcatheter aortic valve implantation (TAVI). She had severe aortic stenosis (mean aortic valve pressure gradient 61mmHg, maximum aortic valve pressure gradient 111mmHg) with mild left ventricular systolic dysfunction. She was a life-long smoker with pulmonary function testing revealing an FEV1 1.48L. Electrocardiography demonstrated sinus rhythm with right bundle branch block and left axis deviation. Cardiac computed tomography was performed to assess suitability for TAVI.
QUESTION
What one of the following would confirm a diagnosis of severe aortic stenosis?
ANSWER C
Calcium score thresholds (men 2065 Agatston Units, women 1274 Agatston Units) differentiate moderate from severe AS [1]. Echocardiographic parameters of severe aortic stenosis (AS) include an increased aortic valve velocity (aortic valve maximum velocity 4 m/sec, maximum pressure gradient 64 mmHg (4v2), mean pressure gradient 40 mmHg). When cardiac output is reduced (i.e. LV systolic dysfunction), a velocity ratio of 4:1 (<0.25) is suggestive of severe AS. A delayed time to peak velocity (>100 msec) represents the late systolic murmur audible on auscultation that is associated with severe AS. [2] Valvulo-arterial impedance >5 mmHg/mL/m2 estimates the LV systolic load suggestive of severe AS and can be useful in patients with hypertrophied,small cavity left ventricles [3].
QUESTION
What features should be considered in this case?
A. Absolute indication for surgical bioprosthesis
B. Increased risk of left coronary artery obstruction
C. Increased risk of high-grade atrioventricular conduction block
D. Increased risk of annular rupture
E. Decreased risk of paravalvular regurgitation
ANSWER D
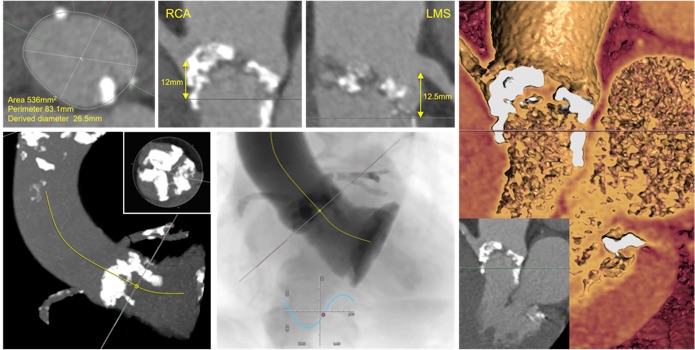
The correct answer is increased risk of annular rupture. This patient has a trileaflet aortic valve with severe calcification involving all three cusps. A nodule of calcification extends along the left ventricular outflow tract (LVOT) following the course of the aorto-mitral curtain. The coronary arteries arise from their normal aortic sinus with an adequate height from the annulus to the coronary ostia. There is extensive macrocalcification of the anterior (right coronary) aortic valve leaflet which may potentially obstruct the right coronary ostium during TAVI deployment. The extensive LVOT calcification beneath the left coronary artery may penetrate the myocardium and either result in acute rupture of the left ventricular free wall or slowly progress to pseudoaneurysmformation. [4]
Clinical practice guidelines would recommend transfemoralTAVI over surgical aortic valve replacement in this individual. [5] Pre-existing right bundle branch block and increased left coronary cusp calcification are independent risk factors for permanent pacemaker implantations post-TAVI. [6] The shallow sinus of valsalva height would preclude use of a self-expanding valve. [7] Extensive aortic valve calcification is associated with post-procedural prosthesis eccentricity and severity of paravalvular regurgitation [8].
QUESTION
If annular rupture were to occur, which location is most likely?
ANSWER A
The native annulus is severely calcified and prominent calcified shards can protrude through the annulus following balloon-expansion TAVI. Small tears or localised rupture may be ‘contained’ by the transcatheter valve preventing further haemodynamic compromise [4]. Subannular ruptures (free myocardial wall, non-coronary cusp, interventricular septum) occur when LVOT calcification disrupts the aortic-outflow tract continuity. Free myocardial wall rupture is located beneath the anterior portion of the left coronary cusp resulting in catastrophic haemorrhage. Perforation below the non-coronary sinus with calcification in the aorto-mitral continuity can generate large shunts into the left atrium. Interventricularseptum perforations may only be detected due to presence of a new conduction abnormality, however haemotoma formation and ventricular septal defects may occur. Supra-annular ruptures may develop in shallow sinuses with injuries to the sinotubular junction or coronary ostia.
Transfemoral TAVI using a 26mm Sapien 3 prosthesis was performed. Following the procedure there was only trivial paravalvular leak and no alteration to atrioventricularconduction.
References
[1] Clavel MA, Pibarot P, Messika-Zeitoun D, et al. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: results of an international registry study. J Am Coll Cardiol. 2014;64:1202-1213.
[2] Kamimura D, Hans S, Suzuki T, et al. Delayed time to peak velocity is useful for detecting severe aortic stenosis. J Am Heart Assoc. 2016;5: e003907 doi: 10.1161/JAHA.116.003907.
[3] Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr. 2009;10:1-25.
[4] Pasic M, Unbehaun A, Buz S, et al. Annular rupture during transcatheter aortic valve replacement: classification, pathophysiology, diagnostics, treatment approaches, and prevention. JACC Cardiovasc Interv. 2015;8:1-9.
[5] Vandvik PO, Otto CM, Siemieniuk RA, et al. Transcatheter or surgical aortic valve replacement for patients with severe, symptomatic, aortic stenosis at low to intermediate surgical risk: a clinical practice guideline. BMJ 2016;354:i5085. Doi:10.1136/bmj.i5085.
[6] Fujita B, Kütting M, Seiffert M, et al. Calcium distribution patterns of the aortic valve as a risk factor for the need of permanent pacemaker implantation after transcatheteraortic valve implantation. Eur Heart J Cardiovasc Imaging. 2016;17:1385-1393.
[7] Ribeiro HB, Webb JG, Makkar RR, et al. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol. 2013;62:1552-1562.
[8] Bekeredijan R, Bodingbauer D, Hofmann NP, et al. The extent of aortic annulus calcification is a predictor of postprocedural eccentricity and paravalvular regurgitation: a pre- and postinterventional cardiac computed tomography angiography study. J Invasive Cardiol. 2015;27:172-180.