Dr Chrysovalantou Nikolaidou1, Dr Andrew D Kelion1
1Radcliffe Department of Medicine, Division of Cardiovascular Medicine, John Radcliffe Hospital, Oxford.
Case history
A 92-year-old woman presented for gated cardiovascular computed tomography for assessment of the aortic annulus and root, coronary arteries and peripheral vascular access prior to Transcatheter Aortic Valve Implantation (TAVI). She had been diagnosed with severe aortic valve stenosis during a routine pre-operative check for treatment of bladder tumours. Her previous medical history included permanent pacemaker implantation for symptomatic second degree atrioventricular block, and paroxysmal atrial fibrillation.
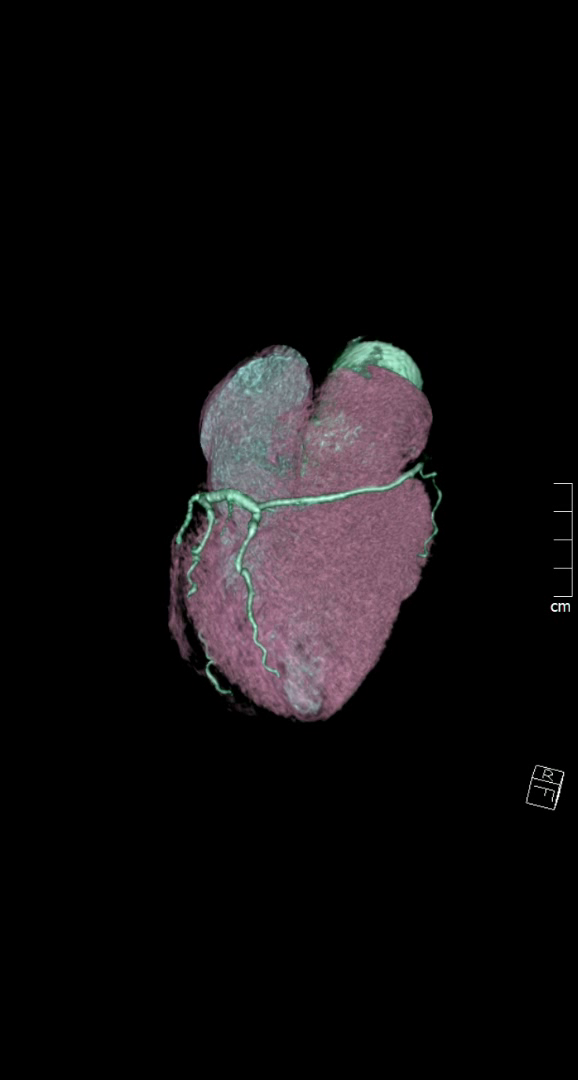
The CT coronary angiogram revealed very unusual coronary anatomy, with complete absence of the left circumflex artery (LCx) (Figure 1, Video 1). There was a huge dominant right coronary artery (RCA), supplying the posterior descending artery and then continuing around the atrio-ventricular groove as a large posterolateral branch to the lateral left ventricular wall. The left coronary artery / left anterior descending artery (LAD) had a normal origin and course (Video 2). There was calcified and mixed plaque disease in both coronary arteries, but no significant luminal stenosis.
Figure 1. Huge right coronary artery supplying the posterior descending artery and then continuing as a large posterolateral branch to the lateral left ventricular wall, as seen on multiplanar reformation (A), maximum intensity projection (B, C) and 3D-volume rendered (D-F) images (white arrows). No left circumflex artery is seen in the left atrio-ventricular groove. The left coronary artery / left anterior descending artery has normal origin and course (white arrowheads), as seen on 3D reconstruction of the coronary artery tree, from the anterior (E) and posterior (F) aspect of the aortic root. Pacemaker wires are also seen in Panel D (white arrowhead).
Video 1: Absence of LCx artery
Video 2: LCA and LAD with a normal anatomy
Questions
- Which is the most common congenital coronary artery anomaly?
- Congenital absence of the LCx
- Origin of the circumflex artery from the RCA or right sinus of Valsalva
- Congenital absence of the RCA
- Anomalous RCA originating from the LAD or LCx
Answer: B
- Which is the most common clinical presentation of coronary artery anomalies?
- Chest pain
- Sudden cardiac death
- Heart failure
- Asymptomatic / incidental finding
Answer: D
- Congenital absence of the LCx:
- Is generally a benign condition
- Has been associated with sudden cardiac death
- Is a very common congenital coronary artery anomaly
- Is an extremely rare anomaly of the coronary arteries
Answers: A, D
Discussion
Congenital coronary artery anomalies may involve the origin, course, and/or structure of the coronary arteries. Their estimated prevalence varies from less than 1% of the general population to 5.8% in the most recent studies using advanced cardiac imaging (1). Most of them are diagnosed incidentally on imaging studies. The most common coronary artery abnormality, excluding separate ostia of the LAD and LCx, is anomalous origin of the circumflex artery from the RCA or right sinus of Valsalva (2). Congenital absence of the LCx is an extremely rare anomaly of the coronary arteries, with an estimated incidence of less than 0.003% to 0.0067% (3, 4). Patients with an absent LCx usually have a large, super-dominant RCA, which supplies blood to the areas of the myocardium usually supplied by the LCx (5). Other concomitant congenital coronary artery anomalies have also been described. Absence of LCx is generally a benign condition. The most common presenting symptom in the majority of cases described in the literature was exertional chest pain. No cases of sudden cardiac death have been reported (3).
Cardiac CT can reliably diagnose congenital coronary artery anomalies, including absence of the LCx, which can be misdiagnosed as ostial occlusion on conventional coronary angiography.
References
- Pérez-Pomares JM, de la Pompa JL, Franco D, Henderson D, Ho SY, Houyel L, et al. Congenital coronary artery anomalies: a bridge from embryology to anatomy and pathophysiology—a position statement of the development, anatomy, and pathology ESC Working Group. Cardiovascular Research. 2016;109(2):204-16.
- Yuksel S, Meric M, Soylu K, Gulel O, Zengin H, Demircan S, et al. The primary anomalies of coronary artery origin and course: A coronary angiographic analysis of 16,573 patients. Exp Clin Cardiol. 2013;18(2):121-3.
- Fugar S, Issac L, Okoh AK, Chedrawy C, Hangouche NE, Yadav N. Congenital Absence of Left Circumflex Artery: A Case Report and Review of the Literature. Case Rep Cardiol. 2017;2017:6579847.
- Shaikh SSA, Deshmukh V, Patil V, Khan Z, Singla R, Bansal NO. Congenital Absence of the Left Circumflex Artery With Super-Dominant Right Coronary Artery: Extremely Rare Coronary Anomaly. Cardiol Res. 2018;9(4):264-7.
- Rawala MS, Ahmed AS, Iqbal MA, Iqbal A, Budde PK, Rizvi SB. Congenital anomaly of coronary artery: absence of left circumflex artery. Journal of Community Hospital Internal Medicine Perspectives. 2019;9(2):140-2.


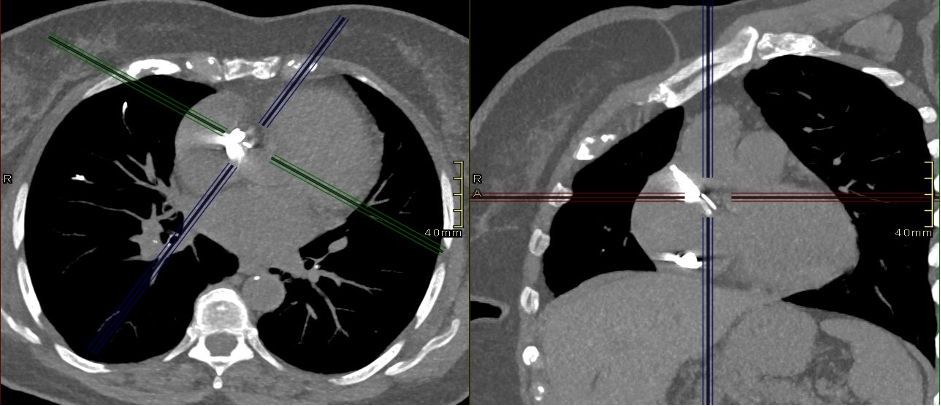
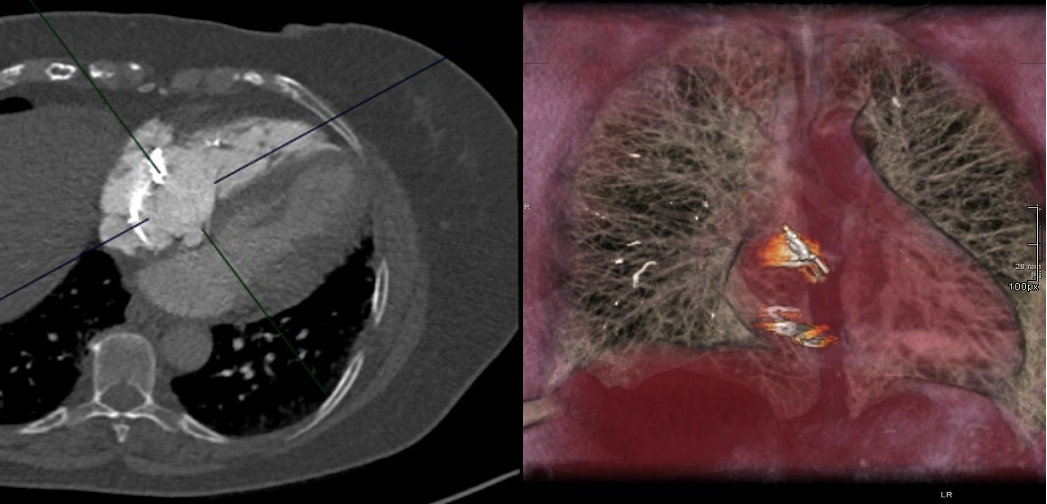
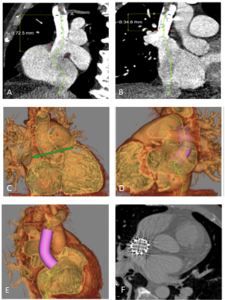
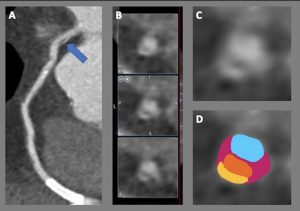
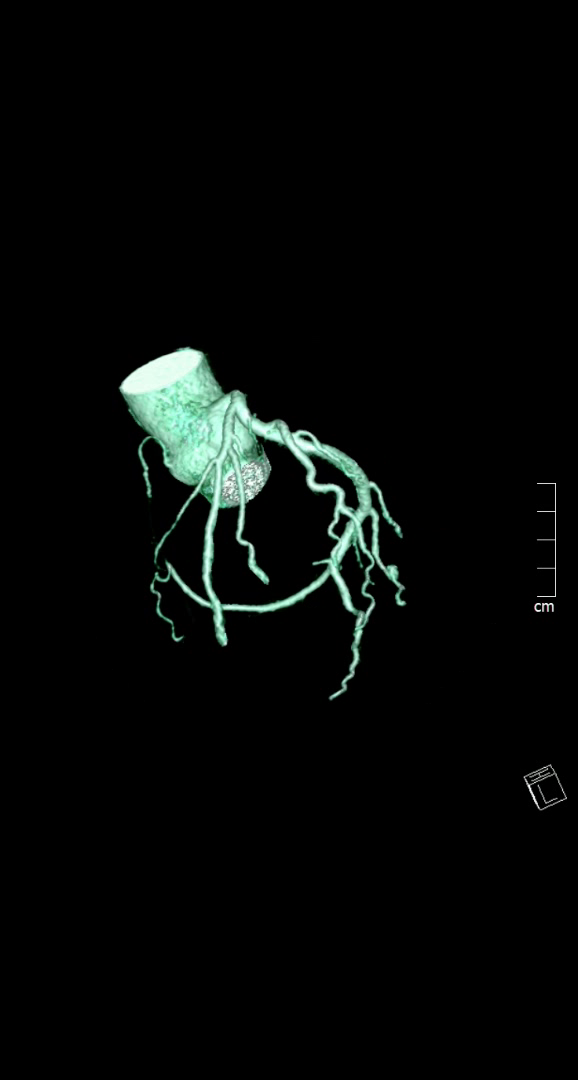
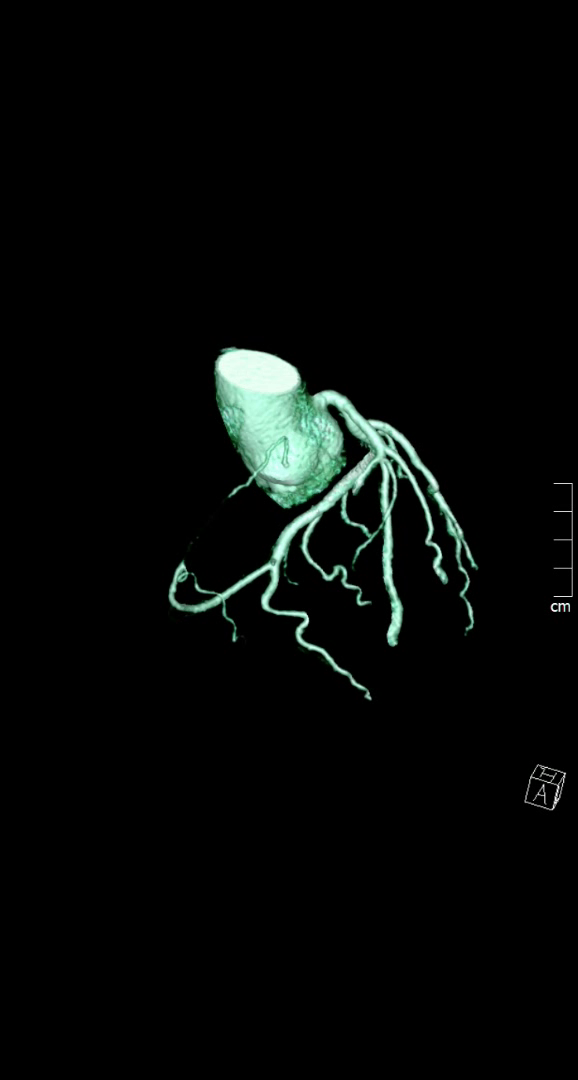
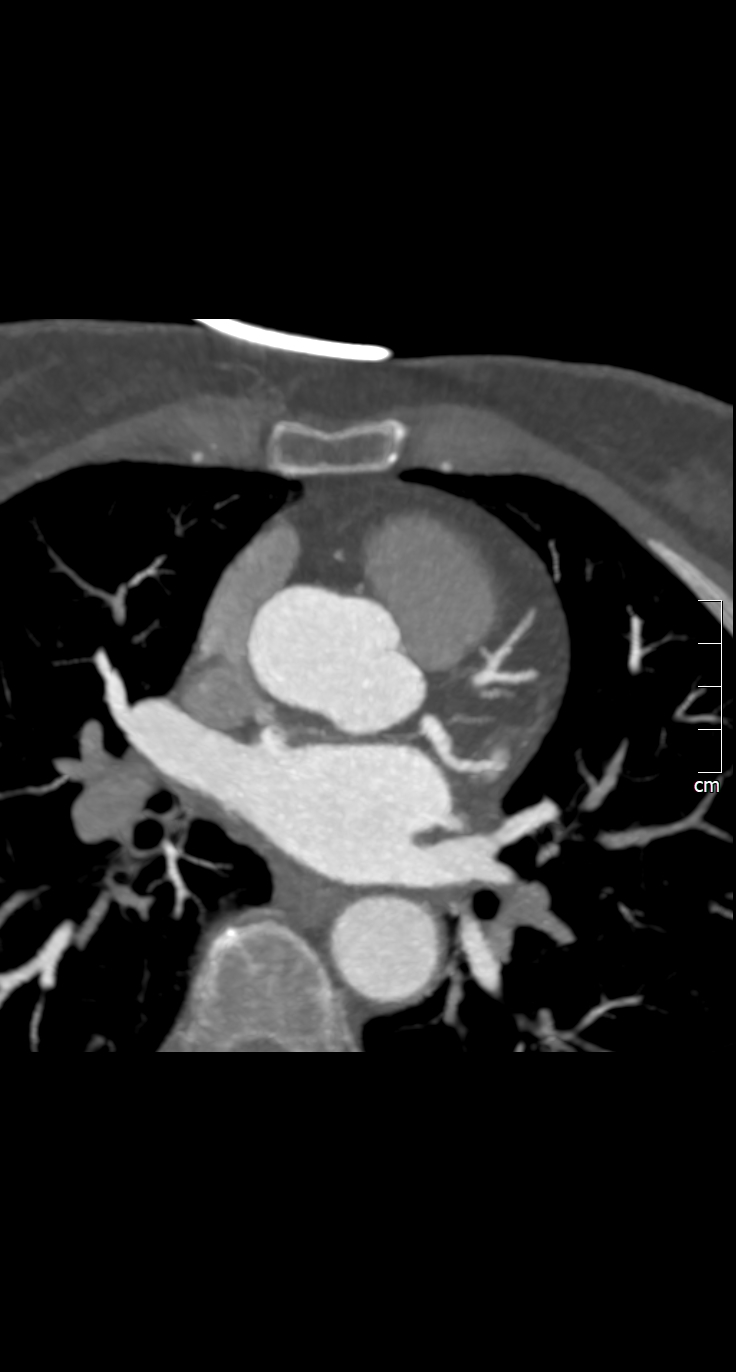 Image 3- Nodal artery arising from LCX
Image 3- Nodal artery arising from LCX